
The impact of current inflation rates, the consolidation of organizations within the healthcare & pharmaceutical industries, and pharmaceutical price implications on U.S. government health programs such as Medicare and Medicaid have brought the Bayh-Dole Act back into the headlines of innovation industry news. This has led to another review of the core purpose of the Act, current administration’s impact with these efforts 1, and the impact that U.S. government pressure on pharmaceutical cost regulation will have on the continued successful growth of innovation across many industries. The recent administration’s statement is focused on the diversification of licenses to third parties for products developed using federal funds–research funded by taxpayer dollars–to combat the consolidation and monopolization of the health care markets, promote equitable access to lower-priced drugs that were/are taxpayer-funded, improving healthcare transparency, and lower overall healthcare costs.
But how does this affect innovation across other industries?
While current controversy of the recent administration’s statement is specific to the healthcare and pharmaceutical sectors, The Bayh-Dole Act, officially known as the “University and Small Business Patent Procedures Act,” was enacted in 1980 in the United States with the purpose of reigniting U.S. government-funded research and innovation developed through non-profits, educational institutions, and small businesses across all industries. Prior to the Bayh-Dole Act, the U.S. government typically retained ownership of inventions resulting from federally funded research. The Act allows not-for-profits, small businesses, and institutions to own and control the IP generated from federally funded research, providing them with the autonomy to make decisions about licensing and commercialization. It helped change the landscape of intellectual property management by incentivising greater engagement with the private sector and has since been revised multiple times2 since its enactment.
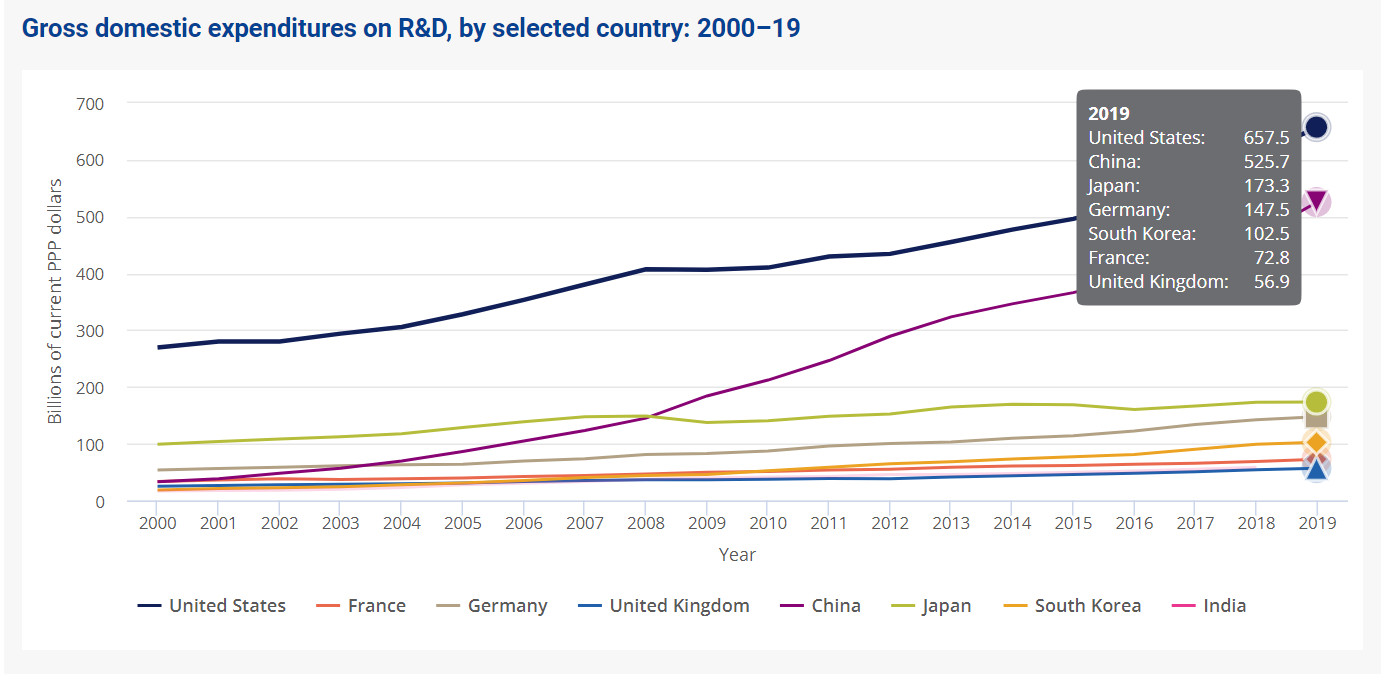
While refusing or eliminating government involvement in R&D across industry sectors may seem like the logical, reactionary (albeit extreme) response to U.S. government pressure on the healthcare sector’s IP ownership, the fact remains that the U.S government leads the world in R&D expenditures, investing $657.5 billion in R&D expenditures in 2019 (leading the world at 27% of global R&D), and is the second largest contributor to global R&D growth worldwide3. While innovations patented through government-funded research represents a fraction of that total, the Act only reinforces innovation across institutions, not-for-profits, and small businesses by granting access to these government assets.
Across Academia, The federal government has long been the largest funder and provided more than half (53%, or around $45 billion) of total funds in 2019. Six departments or agencies provided more than 90% of federal support for academic R&D. Without the Act providing a funding source for R&D across Academia, it would reduce the innovation impact the U.S has on the global market.
How to Best utilize R&D funding and Maximize R&D Innovation Workflows
Regardless of current administration pressures on the healthcare and pharmaceutical industries, it remains clear that the Bayh-Dole Act as a whole continues to foster diverse innovation by providing institutions, not-for-profits, and small business ongoing government funding.

While R&D funding is one of the most critical elements of the R&D process–without which, implementing research wouldn’t even be possible–optimizing team productivity, patent research, competitive analysis, and overall innovation workflow is critical to maximizing research funding. The IP Suite, a robust set of software solutions and analytics that offer competitive data analytics, streamline innovation workflows from ideation to application, and maximize costs, delivers all of the secure, trusted, and comprehensive innovation, ideation, and analytics power to meet your needs.

